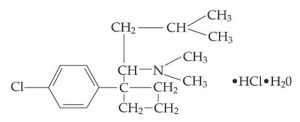
Meridia 15mg is an orally administered agent for the treatment of obesity. The generic name of Meridia is Sibutramine hydrochloride monohydrate. Chemically, the active ingredient is a racemic mixture of the (+) and (-) enantiomers of cyclobutane methanamine, 1-(4-chlorophenyl)-N,N-dimethyl-α-(2-methylpropyl)-, hydrochloride, monohydrate, and has an empirical formula of C17H29Cl2NO. Its molecular weight is 334.33.
The structural formula of Sibutramine hydrochloride monohydrate is shown below.
The structural formula of Sibutramine hydrochloride monohydrate
Meridia 15mg is used in the treatment of obesity and belongs to the drug class anorexiant. Risk cannot be ruled out during pregnancy. Meridia is classified as a Schedule 4 controlled substance under the Controlled Substance Act (CSA). It affects chemicals in the brain that affect weight maintenance.
Meridia is used together with diet and exercise to treat obesity that may be related to diabetes, high cholesterol, or high blood pressure.
Precaution to intake Meridia 15mg:
Meridia 15mg medicine is a compound of highly reactive components and it was withdrawn from the U.S. market in October 2010. Moreover, if the patient has consumed an MAO inhibitor such as furazolidone (Furoxone), isocarboxazid (Marplan), phenelzine (Nardil), rasagiline (Azilect), selegiline (Eldepryl, Emsam), or tranylcypromine (Parnate) in the last 14 days then serious life-threatening side effects can occur in case the patient uses Meridia before the MAO inhibitor has completely clear from the patient’s appetite. Also, the patient should not take Meridia in case of allergic to sibutramine, or have severe or uncontrolled high blood pressure, an eating disorder (anorexia or bulimia), taking stimulant diet pills, or has a history of coronary artery disease, stroke, or heart disease.
Moreover, patients should avoid or take consultation from a medical professional before intake Meridia 15mg if they patient is suffering from the following diseases.
- Severe or uncontrolled hypertension (high blood pressure);
- An eating disorder (anorexia or bulimia);
- history of coronary artery disease (atherosclerosis);
- A history of heart disease (congestive heart failure, heart rhythm disorder);
- A history of heart attack or stroke; or
- If the patient is taking stimulant diet pills.
Moreover, Meridia 15mg is FDA pregnancy category C medicine. And it is unknown whether Meridia will harm an unborn baby. Moreover, while using this medicine a pregnant woman is recommended to inform the concerned doctor before intaking Meridia. It is not known whether sibutramine passes into breast milk or if it could harm a nursing baby.
Furthermore, a few other diseases need to be taken care of and consulted with the doctor before initiating the dosage of Meridia 15mg.
These medical conditions are given below,
- Glaucoma;
- High blood pressure;
- Liver disease;
- Kidney disease;
- Depression;
- Underactive thyroid;
- Epilepsy or seizure disorder;
- A bleeding or blood clotting disorder;
- A history of gallstones; or
- If the patient is older than 65 or younger than 16.
The shape of the Meridia 15mg tablet:
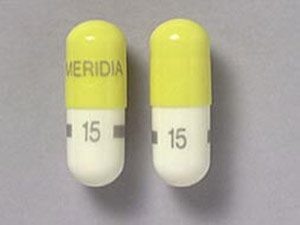
Pills of Meridia pill imprint with 15 MERIDIA on top of the tablet. The color of the tablet is Yellow/White and the shape of the Tablet is Oblong the generic name of the medicine is Sibutramine hydrochloride monohydrate.
The picture of the pill of Meridia 15mg is given below.
Picture of the Meridia 15mg pill
Generic name: Sibutramine hydrochloride monohydrate
Imprint: 15 MERIDIA
Strength: 15 mg
Colour: Yellow/White
Size: 18.00 mm
Shape: Oblong
Availability: Prescription only
Dosage of Meridia 15mg:
The initial dosage can be started from 10 mg which can gradually increase up to 15 mg. The recommended dosage of the medicine is once in a day with or without having appetites. Dosage titration can be only done by the prescription of the doctor after closely monitoring the blood pressure and heart rate changes of the patient. Moreover, dosage titration can be only 4 weeks of the course of the medicine.
Moreover, an Analysis of numerous variables has indicated that approximately 60% of patients who lose at least 4 pounds in the first 4 weeks of treatment with a given dose of MERIDIA in combination with a reduced-calorie diet lose at least 5% (placebo-subtracted) of their initial body weight by the end of 6 months to 1 year of treatment on that dose of MERIDIA. Conversely, approximately 80% of patients who do not lose at least 4 pounds in the first 4 weeks of treatment with a given dose of MERIDIA do not lose at least 5% (placebo-subtracted) of their initial body weight by the end of 6 months to 1 year of treatment on that dose.
side effects of Meridia 15mg:
Meridia 15 mg tablet is a highly chemical reactive medicine. Side effects occur because of misuse and overdose of the medicine. The Prevention of the side effects of Meridia is to take the medicine as prescribed by the doctors. Side effects of the initial dosage may decrease after using the medication for a while.
Moreover, Meridia 15mg is well-tolerated. Common side-effects of the medicine are given below.
- Constipation
- Insomnia
- Dry Mouth
- Abdominal Pain
- Chest Pain
- Anxiety
- Joint Pain
- Excitation
- Dizziness
- Drowsiness
- Irregular Or Painful Menstrual Periods
- Flu-Like Syndrome
- Nausea
- Palpitations
- Tingling Of The Extremities
- Sore Throat
- Sinus Congestion
Summary
Meridia 15mg is used together with diet and exercise to treat obesity that may be related to diabetes, high cholesterol, or high blood pressure. It belongs to the drug class anorexiants. Risk cannot be ruled out during pregnancy. Meridia 15mg is classified as a Schedule 4 controlled substance under the Controlled Substance Act (CSA). It affects chemicals in the brain that affect weight maintenance. Meridia was withdrawn from the U.S. market in October 2010.
Reviews
There are no reviews yet.