Carisoprodol (Soma) Side effects, Dosage & Treatment
Description of Carisoprodol (Soma)
Soma (Carisoprodol) is prescribed as a pain reliever that has an opioid nature and is classified as a synthetic opioid. It operates by controlling the central nervous system (CNS) to disconnect the signals to the brain. It prevents the formation of a molecule that causes pain (prostaglandins). The generic name of Soma is carisoprodol.
Soma dosage 500 mg (carisoprodol) is a muscle relaxant that is used in conjunction with rest and physical therapy to provide relief for up to two or three weeks. It operates by interrupting nerve impulses (pain sensation). Soma’s precise method of action is unclear. It may act by modifying communication between neurons in the brain’s pain-controlling region and those in the spinal cord.
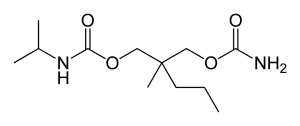
The IUPAC name of carisoprodol is (RS)-2-{[(aminocarbonyl)oxy]methyl}-2-methylpentyl isopropyl carbamate and the chemical formula is C12H24N2O4 with a molecular weight of 260.334 g/mol.
The chemical structure of carisoprodol (C12H24N2O4) is given below.
Chemical structure of carisoprodol (C12H24N2O4)
Carisoprodol is a narcotic analgesic combination medication that belongs to the narcotic analgesic drug class. The risk factor can’t be ruled out throughout pregnancy and its aftermath. The infant may be affected by the medicine’s side effects. carisoprodol is a pain reliever that helps to relax muscles. Because it is widely used to treat muscle spasms and is available in generic form, it is not recommended to use carisoprodol for a long time.
Precaution and contraindication of Soma (carisoprodol)
Soma (carisoprodol) is a muscle relaxant antispasmodic belonging to the family of the drug class of narcotic analgesics. Soma (carisoprodol) is restricted from being classified as a Controlled substance under the Schedule 2 Controlled Substance Act (CSA). Carisoprodol is a powerful pain reliever that can be harmful if used incorrectly or in excess. Moreover, if patients are allergic to Carisoprodol, the patient should consult with the doctor before initiating the dosing of Soma 500 mg tablets.
Moreover, Soma (carisoprodol) may contain inactive ingredients, which can cause allergic reactions and other problems as well. Additionally, if a patient is having a medical history of the following diseases they should inform the concerned doctor immediately before taking the prescription of Soma.
- If the patient is suffering from liver disease
- If the patient has epilepsy or fits that your current treatment isn’t controlling
- If the patient has a history of serious poisoning from sleeping pills, alcohol, pain medicines, or mood-altering substances, the doctor should be consulted.
- If the patient is taking antidepressants or drugs for Parkinson’s disease
Soma dosage of 500mg may cause drowsiness or sleepiness in the patient. As a result, intake of alcohol, marijuana, and cannabis is restricted before or after taking this medicine. Since it might make the patient dizzy and sleepy. It is also recommended that patients should avoid doing any mind-attentive work such as driving or anything which is full mind attention.
Dosage of Soma (carisoprodol)
Soma dosage of 500mg (carisoprodol) should be taken only as prescribed by the medical professional. Moreover, overdose and misuse can cause addiction or death of the patient. Soma (carisoprodol) is an opioid nature of medicine and one should be careful before initiating the dosage of Soma (carisoprodol).
The usual adult dose of carisoprodol for acute pain is 50 to 100 mg orally every 4 to 6 hours. Adults should take 100 mg of carisoprodol once every day for persistent pain. Individual titration is 100 mg for 5 days to forestall side effects and resolve withdrawal symptoms. This could be exaggerated to 300 mg every day.
Commence with a complete daily ER indefinite-quantity rounded all the way down to consecutive lowest a hundred mg increment orally once every day if the patient is presently on the Immediate-Release (IR) carisoprodol. Stop victimizing the other opioid medications round the clock before commencing medical aid with a hundred mg ER orally once every day. to forestall adverse reactions, people square measure titrated in a hundred mg increments every five days till they reach an efficient dose. it’s the potential to require a maximum quantity of three hundred mg every day.
Furthermore, the lowest effective dose of carisoprodol ought to be wont to begin the treatment. In addition, the most generic dose per day ought to be three hundred mg. it is additionally not suggested for kids over the age of 17 or pregnant girls. The drug will pass via breastfeeding and put infants in danger of changing into physiologically dependent on that.
Side effects of Carisoprodol
In the case of misuse or overdose following unintended side effects may appear.
- Agitation
- Irritability
- Depression
- Temporary loss of consciousness
- Tachycardia
- Hypotension
- Extreme weakness
- Cervical spine injury
- Difficulty speaking
- Temporary loss of vision
- Double vision
In the case of serious symptoms or side effects, patients should immediately consult with medical professionals.
Conclusion
Soma 500 mg (carisoprodol) is a muscle relaxant that is used in conjunction with rest and physical therapy to provide relief for up to two or three weeks. It operates by interrupting nerve impulses (pain sensation). Soma’s precise method of action is unclear. It may act by modifying communication between neurons in the brain’s pain-controlling region and those in the spinal cord.
Moreover, to the family of the drug class of narcotic analgesics. Soma (carisoprodol) is restricted from
being classified as a Controlled substance under the Schedule 2 Controlled Substance Act (CSA). Carisoprodol is a powerful pain reliever that can be harmful if used incorrectly or in excess. Moreover, if patients are allergic to Carisoprodol, the patient should consult with the doctor before initiating the dosing of Soma.